Introduction
Genomic testing of skin diseases is now in the hands of dermatologists.
Since 2020, genomic medicine for skin diseases has been mainstreamed in the UK so that phenotyping, diagnostic test selection and returning genomic test results are in the scope of routine dermatology care.
In this video, Professor Neil Rajan of Newcastle University discusses some of the ways in which genomics is currently being applied to improve health outcomes for patients presenting with skin conditions, and how dermatologists can build their genomics knowledge in order to further the cause of mainstreaming genomic medicine across the NHS.
What do I need to know?
Why is genomics important in dermatology?
Although more than 800 skin diseases with genetic causes have now been identified, many patients still do not have a secure genomic diagnosis – and are likely to face quite a journey to reach one.
As skin diagnostic experts, dermatologists are well placed to decide which genomic tests are required for specific patient presentations. To that end, since 2020 dermatologists have had the ability to request testing for certain skin conditions via the National Genomic Test Directory. This means that they also hold responsibility for interpreting and communicating test results for those conditions. In this way, dermatologists can play an important role in counselling families in which one or more members is affected by a genetic skin condition.
Genomic testing can have a significant impact on clinical management and overall patient outcomes. For example, a genomic diagnosis could provide access to skin cancer and internal malignancy screening, meaning that detection happens earlier, leading to an improved prognosis. In addition, an ever-increasing number of targeted therapies are becoming available for various genetic skin diseases – such as dystrophic epidermolysis bullosa – as well as opportunities to enter clinical trials in, for example, PIK3CA overgrowth spectrum conditions.
How is genomics used in dermatology?
Many skin diseases have an underlying genetic cause, and more links are being made all the time between skin presentations and the genome. By thinking about genomics in their day-to-day clinical practice, a dermatologist may be able to reach a precise genomic diagnosis for their patient – which can significantly alter the course of that patient’s treatment and management, and can have a positive impact on their overall health outcomes.
Below are some specific areas of dermatology in which genomics is currently being used for the benefit of patients and their families.
Neonatal dermatology
When newborns present with extensive skin conditions that are thought to be inherited, such as epidermolysis bullosa, a genomic diagnosis can help clarify what is happening for the patient’s family. It can enable clinicians to ensure that parents are well informed about what to expect from their newborn’s condition, as well as about the various treatment and management options available.
Paediatric dermatology
Some genetic skin diseases that commonly present during childhood have been linked to neurodevelopmental conditions. For example, some young patients who present with extensive skin inflammation and scaling will be found to have a genetic condition called ichthyosis. This condition is now understood to be caused by a range of genetic variants that influence the formation of the outer skin barrier. Ichthyosis is inherited in a number of different patterns, depending on the subtype. Some types of genetic variants – for example those seen in x-linked ichthyosis – may be associated with additional neurodevelopmental conditions, such as autism spectrum disorder and attention deficit hyperactivity disorder (ADHD).
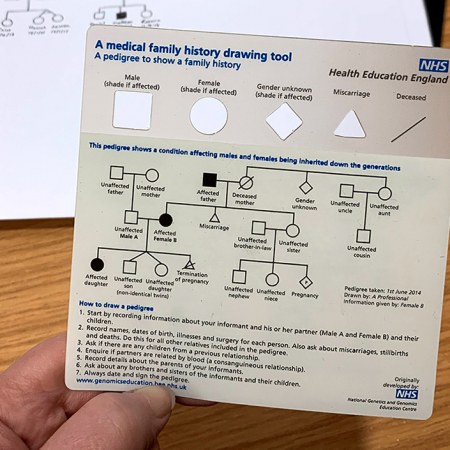
Figure 1: A pedigree showing an x-linked pattern of inheritance in a family affected by ichthyosis
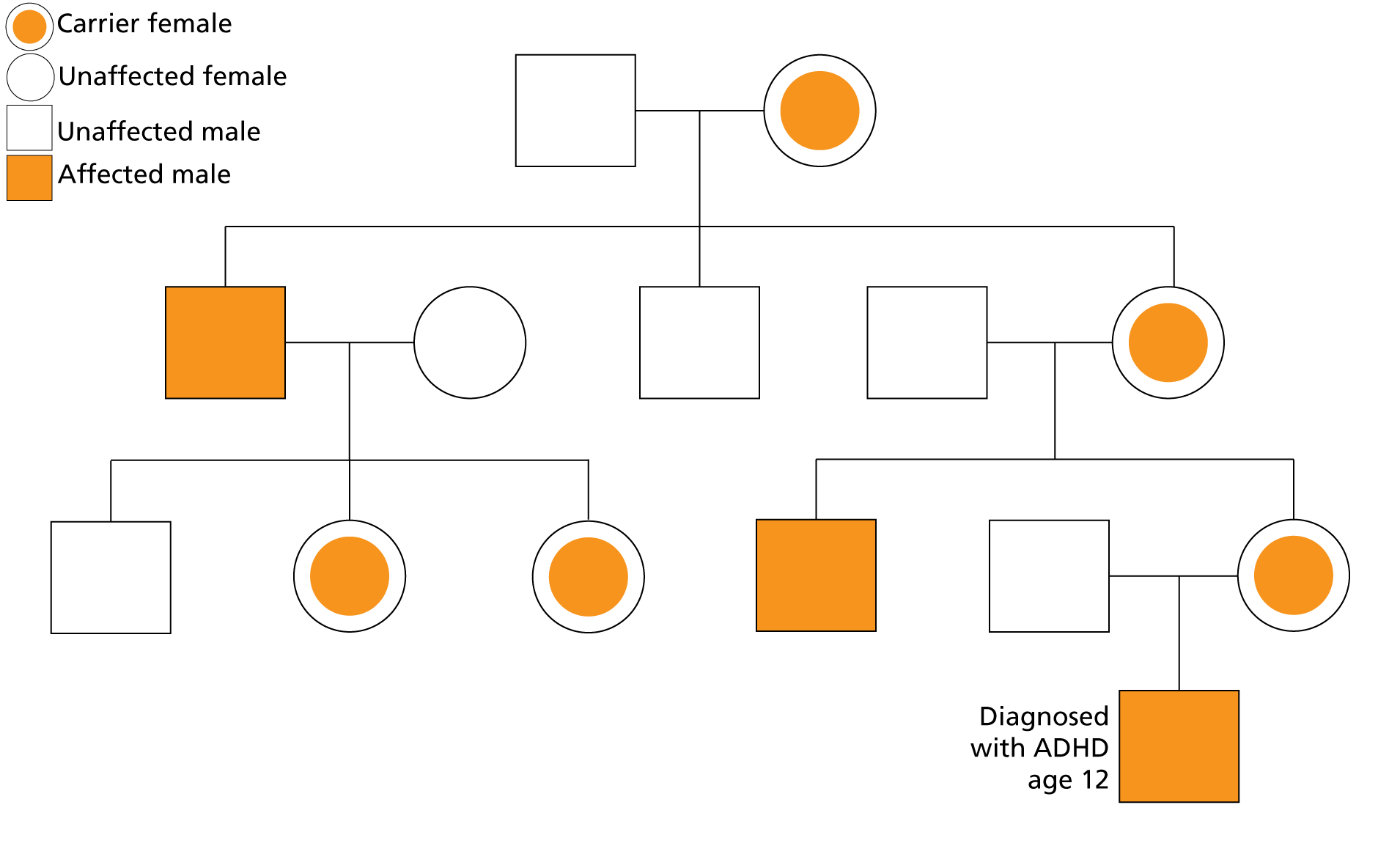
This means that the skin can be an important clue for clinicians, providing insight into additional support that individuals and their families may require.
Skin cancer
Systemic clues from the skin
By examining the skin, dermatologists can decipher valuable clues that may reveal a broad range of underlying genetic causes for patients’ presentations.
Clinical examination of the face, for example, can reveal a potential heritable predisposition to cancer, and is especially relevant in an era of remote consultations. Clinical presentations of certain facial features and cutaneous signs may suggest an underlying genetic predisposition to skin and other cancers; this means that, by identifying them, dermatologists can highlight management and surveillance strategies based on the latest guidelines.
Familial skin cancer and melanoma
In some families, carrying a pathogenic variant in a melanoma-predisposing gene such as CDKN2A can increase the risk of family members developing melanoma. This risk can be passed from generation to generation, and identifying the familial variant can influence clinical decisions around skin surveillance and, in the case of melanoma caused by variants in CDKN2A, cancer screening of the pancreas.
How can I play my part?
Requesting genomic tests
In the UK, dermatologists are able to request genomic tests for skin conditions via the National Genomic Test Directory. This means that, by learning to ‘think genomics’ in their day-to-day clinical practice, a dermatologist can take action to provide patients from all stages of life with a genomic diagnosis that could have a significant impact on their healthcare outcomes.
For a small number of genetic skin conditions, such as epidermolysis bullosa and xeroderma pigmentosum, highly specialised services are in place in certain parts of the country, which receive referrals and include genomic testing as part of the care provided. However, there are many more conditions for which testing is available, but which are not catered for by specialist services – meaning that testing sits with dermatologists.
Watch this short video for some handy hints and tips about requesting genomic testing within the NHS.
The broadening of access to genomic testing to include relevant medical specialties is known as mainstreaming, and it is happening across all specialties in clinical medicine. The tests that are available to dermatologists, which are listed in the National Genomic Test Directory, are designed by a group of clinical experts and include panels of genes relevant to specific dermatology diagnoses, which are updated once a year.
Dermatologists working in clinical practice can develop their own understanding of how to request genomic tests by accessing free online resources on topics such as:
- how to take a family history and draw a family tree;
- how to take informed consent for testing; and
- how to communicate results to patients.
Interpreting results and explaining them to patients
Once genomic testing has been requested and carried out, the results will be analysed by clinical scientists in the genomic laboratory and a report will be produced (this typically takes a few months). The responsibility for interpreting the implications of the report and communicating them to the patient will sit with the dermatologist who requested the test – though support will be available from clinical genetics services, and there may be a local multidisciplinary team (MDT) meeting at which the results can be discussed.
Learn more about reading, understanding and communicating genomic test reports in this video tutorial, and explore the types of results you might find in this short film.
Referring onwards
It is important to remember that not every aspect of genomic medicine in relation to genetic skin conditions sits with dermatologists. Working in partnership with clinical geneticists and genetic counsellors is a crucial part of the dermatologist’s role in genomics mainstreaming within the specialty.
At the point at which a genomic diagnosis is made, key tasks should be handed over to clinical genetics services – including:
- discussing family planning options with the patient; and
- undertaking cascade testing (testing additional members of the patient’s family).
A referral to clinical genetics services should also be made if a genomic diagnosis is suspected but testing has not identified one – for example, if a genomic test report includes a finding of a variant of uncertain significance.
In some NHS trusts, MDTs made up of dermatologists, clinical geneticists and genetic counsellors meet to discuss test results and next steps in patient care.
Further learning and guidance
The mainstreaming of genomic medicine is a central theme in the NHS Long Term Plan, which means that it is now more important than ever for clinicians working across all medical specialties to develop their understanding of what genomics is and how it applies to their practice.
There is a wealth of training and education resources available, from bitesize genomics resources and short online courses that cover the basics to more in-depth courses about genomic testing within the NHS, and the majority are free for NHS employees.
There are also rare disease collaborative networks that can provide expert advice on conditions that impact the skin, such as tuberous sclerosis complex, mosaic skin diseases and PTEN hamartoma tumour syndrome. The Skin Genetics Group, part of the British Association of Dermatologists, also hosts a meeting at the national annual conference during which genetic skin cases are presented and clinically relevant research updates are provided by leading experts.
If you wish to learn more about a particular genetic skin condition, a good place to start is with its dedicated organisation and/or patient advocacy group, if they exist. Some such groups include:
- the Ichthyosis Support Group;
- the Pachyonychia Congenita Project; and
- DEBRA UK.
Case example: Familial melanoma
- A male patient aged 35 is referred by the skin cancer MDT because he has developed a melanoma and has a family history of melanoma.
- His older brother has been diagnosed with metastatic melanoma warranting surgery and immunotherapy, and his mother has already had surgery to remove three melanomas, starting from the age of 30.
Figure 2: A pedigree showing the patient’s known family history
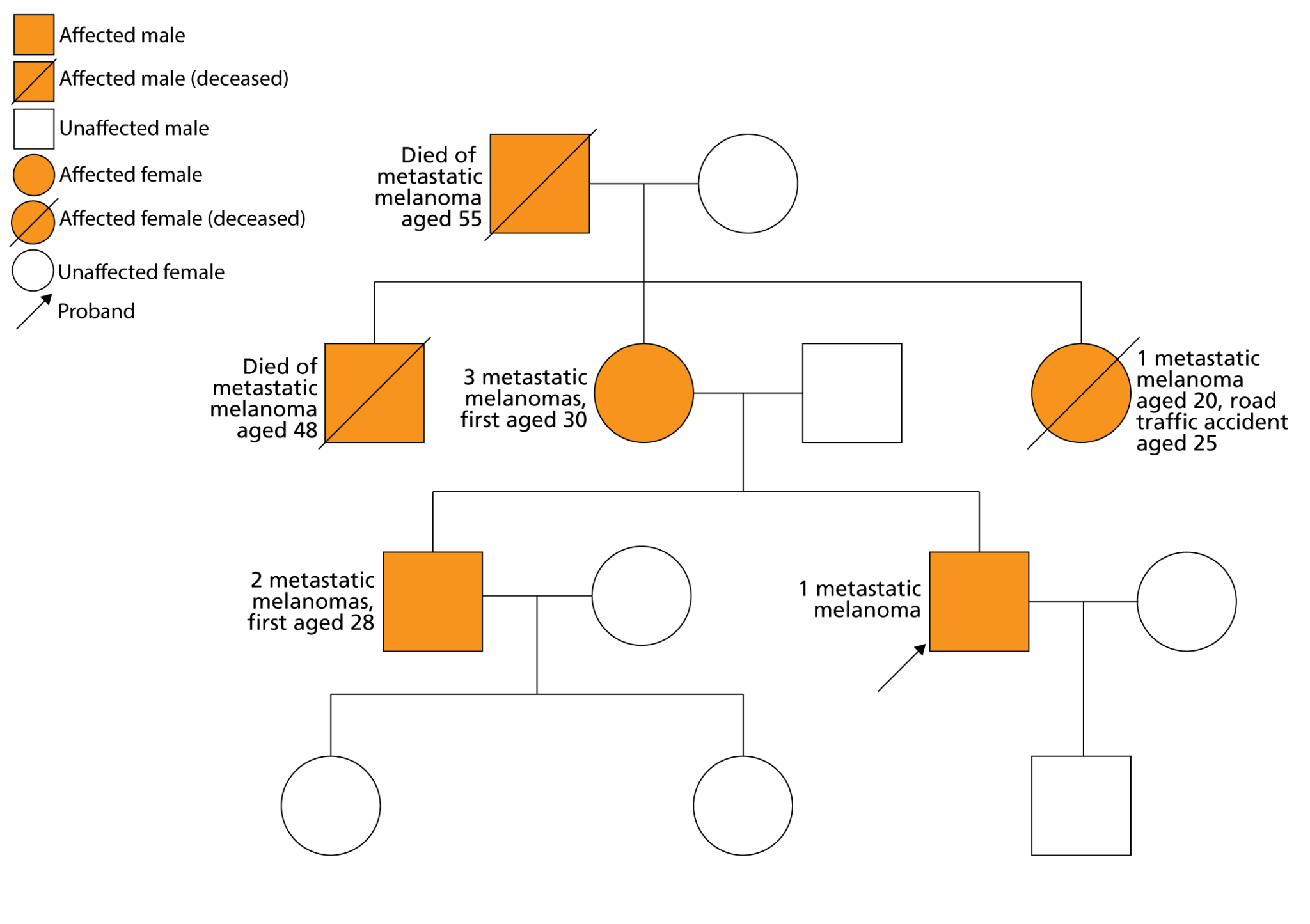
- Suspecting a familial melanoma-predisposing genetic variant, the patient’s dermatologist asks his mother to provide a blood sample for genomic testing.
- Four genes are tested as part of the melanoma gene panel (which sits under the test code R254 in the National Genomic Test Directory), and a variant in CDKN2A is found. As a result, a diagnosis of familial atypical multiple mole melanoma syndrome (FAMMMS) is confirmed. FAMMMS is a condition in which patients can develop multiple atypical moles and melanoma, making it difficult for them to notice changing moles. It is inherited in an autosomal dominant pattern and is known to be associated with carcinomas in other parts of the body, primarily the pancreas.
- The dermatologist refers the case to the local clinical genetics service, and cascade testing is carried out by genetic counsellors. Various family members are also found to have the CDKN2A variant. All those affected are offered skin surveillance and, from the age of 40, endoscopic ultrasound to screen for pancreatic cancer. Those found to be unaffected are advised about sun protection and self-surveillance to monitor any changes in the skin.
- In this case, genomic testing helped identify high-risk individuals within the family, influenced lifestyle choices and raised awareness of the importance of self-examination and early reporting.
Case example: Palmoplantar keratoderma
- A male patient is diagnosed with palmoplantar keratoderma, a condition that features thickening of the soles of the feet and palms of the hands that makes walking very painful and severely limits hand function.
- The patient has received ointments and oral therapies, but continues to experience limitations in function, and is at times forced to crawl rather than walk because of his pain. This is having a significant impact on his quality of life and mental health.
- The patient’s dermatologist requests genomic testing, which reveals a variant in the TRPV3 gene known to cause the rare condition Olmsted syndrome. This diagnosis provides the patient with access to a clinical trial that is seeking to understand more about the function of TRPV3 and attempting to treat patients’ symptoms with erlotinib, an epidermal growth factor receptor inhibitor.
- While the patient is taking part in the clinical trial, the erlotinib treatment leads to a rapid resolution of his pain.
- In this case, genomic testing diagnosed the patient with a specific subtype of palmoplantar keratoderma that gave him access to a clinical trial and a specific treatment, which brought about a dramatic improvement in his symptoms and quality of life.
To find out more about this patient’s story, read this extended article by Genomics England.
Learning opportunities
Teaching aids
Clinical resources
Identifying patients
Activity: Taking and drawing a genetic family history
Equip yourself with the knowledge and skills to construct a genetic pedigree.
Activity: Recognising the clinical clues of a genetic condition
Familiarise yourself with the clinical features of a range of genetic conditions
Communicating with patients
Activity: Returning genomic test results to patients
There are a range of factors to consider when returning genomic results to patients, based on whether there is a confirmed diagnosis, an uncertain result or a negative result.