Examining liquid biopsy in cancer management
Less-invasive method touted as potentially revolutionary for cancer care – could this mean the end for tissue biopsies?
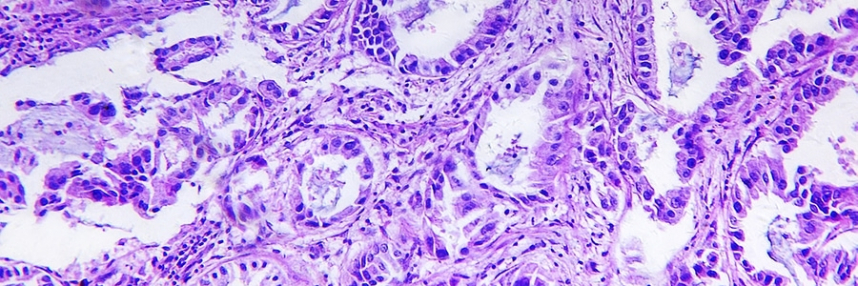
A new technique dubbed ‘liquid biopsy’ could have a major impact on the management of a range of cancers, especially lung cancer. Just as non-invasive prenatal testing (NIPT) utilises fragments of fetal DNA present in maternal blood, liquid biopsy extracts and analyses small fragments of tumour DNA (ctDNA) released into a patient’s bloodstream by tumour cells as they die.
Use in lung cancer
Lung cancer is one of the most common types of cancer and non-small cell lung cancer (NSCLC) accounts for roughly 85% of cases, with most patients presenting with advanced disease at diagnosis. Genetic testing of patients’ solid tumour biopsy samples determines whether they harbour mutations in the EGFR gene and should therefore respond to treatment using drugs called tyrosine kinase inhibitors. This is a classic example of personalised or precision medicine, using a companion diagnostic to see whether or not a treatment is suitable.
However, a solid tissue test is not feasible for around 30% of lung cancer patients, either because they are too ill for the procedure or because it is not possible to obtain suitable biopsy tissue samples. Liquid biopsy opens to the door to determining the optimal treatment for these patients.
Cancer management
As repeat blood samples can be taken with relatively little effort, a liquid biopsy can also be used to monitor emergence of genetic mutations that might confer resistance to treatment. The test is much easier for patients to tolerate compared with repeat solid tissue biopsies, which are rarely carried out in lung cancer patients, allowing long-term personalisation of treatment to their tumour at different points in time.
Liquid biopsy could potentially be used more widely in oncology to monitor the responses to a treatment, revealing the emergence of mutations that might confer resistance to a given therapy and allowing an alternative to be tried promptly. It might similarly be used to monitor for possible disease relapse, and some believe that one day these tests could even be used for screening purposes, identifying cancer in patients before any symptoms are detectable using current methods.
US regulator the Food and Drug Administration recently approved the first liquid biopsy test for EGFR mutations in NSCLC, and the results of the largest study of liquid biopsies to detect cancer mutations to date has reported on over 15,000 tests in patients with different types of cancer.
Improving patient care
Liquid biopsies are an important new option for lung cancer patients where solid tissue biopsies are not feasible, and could in the future form a useful part of the care pathway for other patients too. However, there are limitations; a solid biopsy remains the gold standard technique because liquid biopsy can produce a negative result. Whilst a positive result is actionable, a negative result is considered inconclusive and should be followed up, if feasible, with a solid biopsy. This is because the tumour might be positive for the mutation of interest but because it is not shedding enough DNA into the blood stream the mutation might not be detected by the liquid biopsy test. For example, in the recent study, the liquid biopsy did not show any evidence of the tumour in 15% of the patients.
–