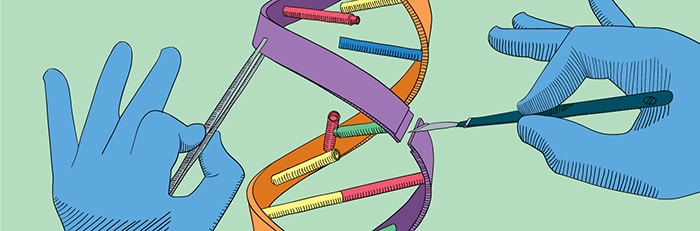
World first with genome edited inside patient’s body
Experimental procedure has potential for treating several metabolic disorders, but the long-term risks are yet to be fully understood
A treatment where genome editing takes place inside the patient’s body is being trialled in California to combat the metabolic disorder Hunter syndrome.
The patient, Brian Madeux, was given an infusion containing billions of DNA copies of the gene that codes for an enzyme he lacks, as well as a type of genome editing machinery called zinc finger nucleases (ZFNs). Previous genome editing treatments have involved altering extracted blood or skin cells in the lab, and then returning the treated cells to the patient.
“We cut your DNA, open it up, insert a gene, stitch it back up. Invisible mending,” Dr. Sandy Macrae, president of Sangamo Therapeutics, which is pioneering the treatment, told the Associated Press. “It becomes part of your DNA and is there for the rest of your life.”
Risk versus reward
The potential outcome is that a single infusion will enable patients to manufacture the missing protein in their own cells, reducing or removing the need for additional treatments. Study leader Dr Paul Harmatz, from UCSF Benioff Children’s Hospital in Oakland, California, estimates that only one per cent of liver cells need to be successfully edited for the patient to make an adequate quantity of the enzyme.
The risks, however, are that the patient’s DNA will be altered in unplanned ways, such as cuts to the DNA in the wrong places. If such cuts occur in critical genes, the patient may develop harmful effects such as developing cancer. Although ZFNs are much more accurate than previous types of gene therapy, unlike cells altered in the lab, there is no way to be certain that there are no off-target effects.
In this case, the patient was prepared to go ahead with the treatment. “I’m willing to take that risk,” Madeux told the AP. “Hopefully it will help me and other people.”
Hunter syndrome
People with Hunter syndrome lack a functional copy of the gene that makes a protein called I2S. Without this enzyme, the body cannot break down complex sugars called mucopolysaccharides and so these molecules accumulate in the body’s cells, causing damage to the brain and many other body systems and tissues.
Madeaux, from Arizona, says he took part in the trial because he is “in pain every second of the day”. He has already undergone 26 operations as a direct result of Hunter Syndrome, including hernia repair, surgeries on his ears, eyes and gall bladder, and to relieve bones pinching his spinal column.
The treatment will not reverse existing damage, but if it works it could replace the current treatment – expensive weekly intravenous infusions of the enzyme – which can ease symptoms but does not prevent brain damage.
From bench to beside
It will take about three months before tests show whether the treatment has been a success. “I’m nervous and excited,” Madeux said. “I’ve been waiting for this my whole life: something that can potentially cure me.”
Following promising results in animal studies, the therapy is being tested for human safety in adults first, but the hope is to treat younger patients in future. “We want to try to address this disease before any of the consequences happen, rather than in adult patients where many of the features are already fixed,” Dr Macrae said.
In the UK, if clinical trials of a new therapy go well, the treatment would still have to clear some regulatory hurdles before being made available to NHS patients. It would first need to be assessed by the European Medicines Agency or the UK’s Medicines and Healthcare products Regulatory Agency to pass strict rules about safety and efficacy.
If these bodies agree to licence the treatment, UK doctors would be allowed to prescribe it. However, a separate assessment by NICE would decide if it should be available on the NHS.
Broader applications
Sangamo Therapeutics is using the same genome editing approach to try treating another metabolic disorders, as well as haemophilia.
Speaking to the Telegraph, Professor Robin Lovell-Badge of the Francis Crick Institute (which was not involved in the trial) said: “There’s a lot of potential for treating liver diseases in this way.”
But he warned that the approach may not be applicable to all genetic conditions: “Taking it to more complex things like muscular dystrophy and cystic fibrosis will require a lot more work.”
–