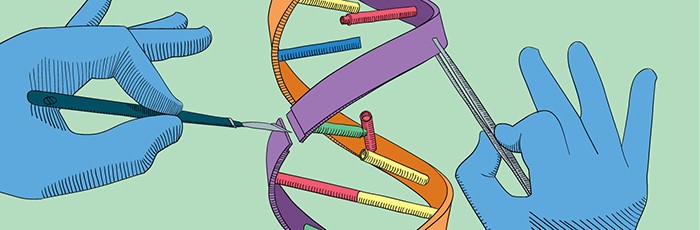
What are genome editing and gene therapy?
Gene therapy and genome editing technologies are increasingly being reported on in the news and discussed in medical literature, but what are they?
There are thousands of rare genetic conditions, with many caused by known variants in single genes. Modern technologies can allow us to treat some of these genetic conditions at the source, either by providing a non-pathogenic (non-disease-causing) variant of the gene or by modifying the existing pathogenic copy. These gene therapy and genome editing technologies are revolutionising the treatment of some of these single-gene conditions.
What is gene therapy?
Gene therapy is a treatment that modifies the genome of specific cells to treat or prevent a genetic condition or disease.
There are several ways that gene therapy can be carried out. A common method is using a viral delivery system called a viral vector. This type of gene therapy delivers a functional copy of a single gene into the cells of a target organ or tissue. It works by packaging a functional gene into a virus, such as an adeno-associated virus or a lentivirus. Then the virus delivers the packaged gene into the patient’s genome.
While this type of gene therapy has had many successes – there are now several approved gene therapies of this type available to patients – there are limitations.
One difficulty is that viral genomes are generally smaller than some human genes. This means the viral package cannot fit in longer human genes, which can be up to thousands of kilobases in length. Furthermore, many viruses can insert their genetic material into the host DNA at seemingly random locations. If this happens, it can disrupt non-target genes, potentially causing harm to the patient.
- Gene therapies in detail: Introduction to gene-directed therapies webinar
What is genome editing?
Genome editing is a type of gene therapy that involves making targeted and deliberate changes to a specific region in the genome. This could be to insert, delete, replace or alter a DNA sequence. It can be carried out completely outside of the body in the laboratory (in vitro), within cells to be re-introduced into a living organism (ex vivo), or in the organism itself (in vivo).
Therapies developed using genome editing technologies are sometimes referred to as genome-editing therapies or CRISPR-therapies. These all share the aim of gene therapy – the treatment of single-gene disorders – but they use a genome-editing approach to alter the native genome sequence instead of providing a new copy of the gene.
Beyond the clinic, genome editing has applications in research, such as disabling working genes in lab cultured cells or model organisms. Researchers often do this to understand the function of a gene by seeing what happens to the cell or organism when the gene of interest is ‘switched off’. The introduction of CRISPR/Cas-based methods has made genome editing affordable and easy to use in the lab.
- Further reading: Five key questions answered in genome editing
Their increasing prominence in medicine
Both gene therapy and genome editing have become increasingly visible in research, in the clinic and in the news.
Libmeldy, a type of gene therapy, recently hit headlines after a 10-month-old baby became the first patient to be given the drug through the NHS. Other gene therapies have been approved in recent years, two examples being voretigene neparvovec (Luxturna) for some retinal dystrophies and onasemnogene abeparvovec (Zolgensma) for spinal muscular atrophy (SMA).
- Find out more: Watch our webinar on SMA therapeutics
Recently, the USA’s Food and Drug Administration and the European Medicines Agency granted fast-track/priority status to a CRISPR-based gene therapy called exa-cel (formerly CTX001). If approved, it will be used to treat sickle cell disease and beta thalassemia. Other genome editing therapies are in clinical trials, but none have been approved (yet) for single-gene disorders. These CRISPR-based therapy approaches have, however, been used to create edited cells for CAR-T cell therapy, as we reported last month.
As researchers and clinicians identify an increasing number of applications for these emerging technologies, the hope is that – as noted in England’s Rare Diseases Action Plan – they will bring life-changing benefits for patients, and their families, with a rare condition.