Description
This course is intended to help healthcare professionals understand key information to consider when offering a genomic test to patients and their family members. The session will cover the processes in place for genomic data and samples that are handled within the NHS GMS.
Why take this course?
This course is designed to present an overview of the types of data and samples that may be used in the GMS throughout the process of requesting, analysing and reporting a genomic test. It forms part of a series of courses that provides best-practice guides to help learners enable informed patient choice about genomic tests and consider practical and ethical aspects to genomic testing.
The series aims to provide you with a foundation for addressing genomic testing with patients, or to refresh your knowledge if you are already doing this in practice.
Who is this course aimed at?
This course is primarily targeted at any healthcare professional working in the NHS who will be involved in requesting targeted genomic tests, based on their specialty area and scope of practice. Individuals accessing this course are expected to already have a fundamental understanding of genomics in relation to their clinical practice.
If you are or will be requesting genomic testing in your clinical practice, it is important that you are aware of any local or regional requirements to evidence your competence to do so. Completing this course may form part of this evidence, and a certificate demonstrating completion can be accessed once you have undertaken the assessment of the course.
What will I learn?
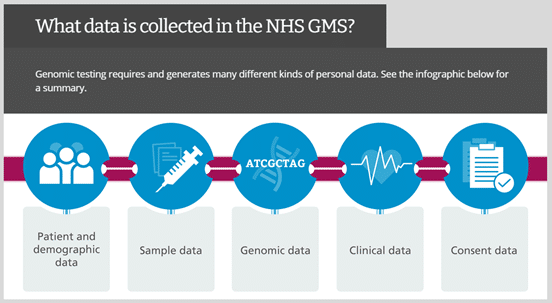
You will learn about how genomic samples and data are stored and accessed in the NHS GMS. This will include any changes and updates from current local arrangements with the introduction of the GMS and whole genome sequencing (WGS) as part of clinical care. You will also learn how this links to genomic research initiatives, particularly the National Genomic Research Library (NGRL).
How will I learn?
You will learn through textual content, images and videos. On completion of the course, a short assessment will provide an opportunity to demonstrate what you have learned. You will then be able to receive a record of your feedback and obtain a downloadable certificate of participation.
Related sessions
To learn more about facilitating genomic testing in the GMS, take a look at these other courses in the Facilitating Genomic Testing series:
- Introduction to Offering Genomic Tests
- The National Genomic Research Library
- Discussing Diagnostic Germline Genomic Tests
- Discussing Targeted Germline Genomic Tests
For introductory information on genetics and genomics browse through our Genomics 101 collection.