Mismatch repair deficiency and microsatellite instability
Mismatch repair deficiency – where errors in DNA base pairing are not routinely corrected – can lead to microsatellite instability, where unrepaired mistakes within repetitive DNA sequences (microsatellites) alter the length of the microsatellite.
Introduction
Microsatellite instability is a feature of around 30% of endometrial cancers and 15% of colon and stomach adenocarcinomas, and is found at lower levels in a variety of other solid tumours. The finding of mismatch repair (MMR) deficiency or microsatellite instability (MSI) may be caused by somatic (tumour) or constitutional (germline) genomic anomalies – the latter of which indicates the presence of Lynch syndrome.
Molecular biology
- Errors known as mismatches, where a guanine base erroneously pairs with a thymine, or cytosine with an adenine, or insertion-deletion loops arising within DNA sequences, may occur due to oxidative stress or slippage during DNA repair. Such errors are usually repaired by the mismatch repair system.
- MMR deficiency is associated with a defect in the DNA mismatch repair machinery.
- MMR deficiency can be assessed by immunohistochemistry, checking for the presence or absence of the MMR proteins, with absence of one or more proteins indicating MMR deficiency. The pattern of MMR protein loss is predictive of the underlying genetic aberration.
- Microsatellites are repetitive DNA sequences. A defective MMR pathway means that errors within microsatellites are unrepaired, which may alter the length of the microsatellite, leading to MSI.
- MSI can be assessed by examining repeat sizes of select microsatellite markers using next-generation technologies. Tumours may be defined as MSI-high (MSI-H) (instability across multiple markers) or MSI-low (MSI-L) (instability at one marker only).
- MMR-deficient tumours usually demonstrate MSI, but these results may be discordant depending on the underlying genotype and technical aspects of testing.
Genomic testing
- NICE guidelines now recommend screening all colorectal cancers and endometrial cancers for evidence of MMR deficiency at first diagnosis in order to identify those patients requiring further testing for Lynch syndrome, as well as to help in treatment planning.
- In colorectal cancers, testing should be undertaken using either:
- MSI (microsatellite instability) testing to identify MSI-H or MSI-L tumours; or
- IHC (immunohistochemistry) to identify loss of expression of MMR proteins (see figure 1).
- In endometrial cancers, testing should be undertaken using IHC to identify loss of MMR proteins. MSI testing is less reliable in endometrial and other extracolonic tumours and is also less reliable if tumour cellularity is low.
- If a colorectal cancer demonstrates loss of MLH1/PMS2 or MSI-L or MSI-H, analysis of the BRAF gene in tumour-derived DNA should be undertaken. The presence of a pathogenic somatic BRAF variant, most commonly V600E, is rare in Lynch syndrome-associated colorectal cancers, and detection of such a variant indicates sporadic MMR deficiency. If a pathogenic BRAF variant is not identified, or if MLH1/PMS2 deficiency is noted in an extra-colonic tumour, MLH1 promoter hypermethylation test should be performed. BRAF testing is not helpful in non-colorectal cancers.
- The majority of MLH1/PMS2-deficient tumours will demonstrate MLH1 promoter hypermethylation in the tumour. A small proportion of Lynch syndrome cases may be caused by constitutional hypermethylation of the MLH1 promoter, with evidence of hypermethylation in the blood as well as the tumour. If neither MLH1 promoter hypermethylation nor a pathogenic BRAF variant is identified, germline testing of the MMR genes should be undertaken to check for Lynch syndrome.
- If immunohistochemistry results are abnormal, showing loss of MSH2 and/or MSH6, or isolated loss of PMS2, the patient should be tested for Lynch syndrome by genomic testing of germline DNA.
- In a small proportion of patients with Lynch syndrome, cancers will not demonstrate microsatellite instability or MMR deficiency. Lynch syndrome may still be suspected from a family history of bowel cancer and other Lynch syndrome-related cancers, or early onset of these cancers (less than 50 years), irrespective of the results of MMR IHC or MSI testing, triggering germline genomic testing of the individual and the family as appropriate.
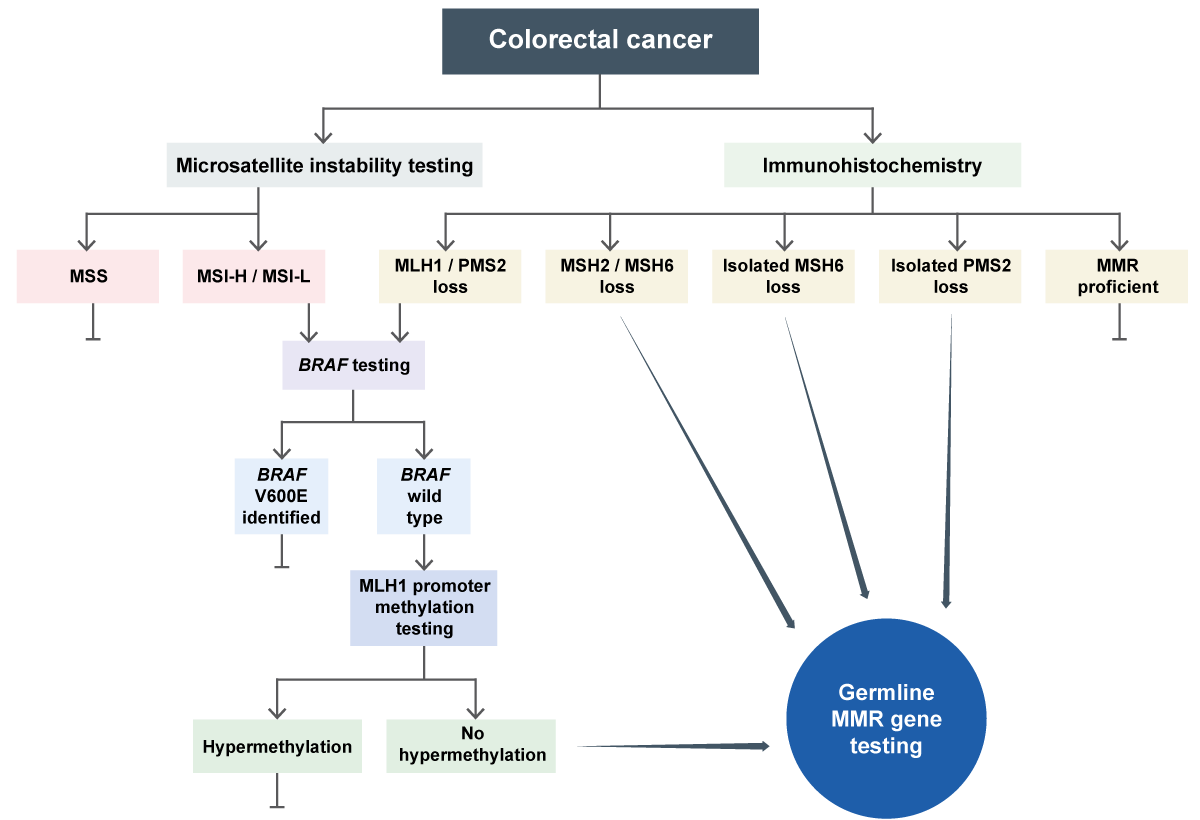
Figure 1: Germline MMR testing in colorectal cancer
Image copyright: Terri McVeigh
Management implications of genomic testing
- The finding of MMR deficiency in solid tumours may permit the use of immunotherapy agents and also impacts the use of chemotherapy in stage II colorectal cancers.
- If testing confirms Lynch syndrome, then genomic testing will need to be cascaded to family.
- In those with a diagnosis of Lynch syndrome:
- Daily aspirin has been shown to reduce the risk of colorectal and certain other cancers.
- Surveillance for colorectal cancer by screening colonoscopy every two years is indicated (from age 25 for MLH1/MSH2 and age 35 for MSH6/PMS2 mutation carriers). (See UKCGG guidelines.)
- Helicobacter pylori testing and eradication should be undertaken, particularly before commencing aspirin chemoprophylaxis.
- Screening or risk-reducing surgery for extracolonic cancers should be considered. Risk-reducing gynaecological surgery is not usually recommended before the age of 35.
- There is evidence demonstrating the beneficial effects of a diet high in resistant starch in managing non-colorectal cancer risks in people with Lynch syndrome. Information should be provided on increasing dietary fibre.
Key messages
- MMR deficiency can be identified by immunohistochemistry, with absence of one or more MMR proteins indicating a deficiency.
- MSI can be assessed by examining repeat sizes of select microsatellite markers using next-generation technologies.
- NICE guidelines recommend screening all colorectal cancers and endometrial cancers for evidence of MMR deficiency at first diagnosis to identify any patients who require further testing for Lynch syndrome, and to help in treatment planning.
Resources
For clinicians
- NHS England: National Genomic Test Directory and eligibility criteria
- NICE: Molecular testing strategies for Lynch syndrome in people with colorectal cancer
- NICE: Molecular testing strategies for Lynch syndrome in people with endometrial cancer
- UK Cancer Genetics Group: Gene specific guidelines for management of patients with Lynch -syndrome
References:
- Bonneville R, Krook MA, Kautto EA and others. ‘Landscape of Microsatellite Instability Across 39 Cancer Types‘, JCO Precision Oncology 2017: volume 1. DOI: 10.1200/PO.17.00073
- Monahan K, Bradshaw N, Dolwani S and others. ‘Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/United Kingdom Cancer Genetics Group‘. British Medical Journal Gut 2020: volume 69, issue 3, pages 411-444. DOI: 10.1136/gutjnl-2019-319915
- Ryan NAJ, McMahon R, Tobi S and others. ‘The proportion of endometrial tumours associated with Lynch syndrome (PETALS): A prospective cross-sectional study‘. PLOS Medicine 2020: volume 17, issue 9. DOI: 10.1371/journal.pmed.1003263
- Syngal S, Brand RE, Church JM and others. ‘ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes‘. The American Journal of Gastroenterology 2015: volume 110, issue 2, pages 223-263. DOI: 10.1038/ajg.2014.435
For patients
- Lynch Syndrome UK
- Royal Marsden NHS Foundation Trust: Beginner’s Guide to Lynch syndrome (PDF, 64 pages)
- UK Cancer Genetics Group: Patient resources