Chorionic villus sampling
Chorionic villus sampling is an invasive clinical procedure performed during pregnancy. It is used to obtain a small biopsy of the placenta in order to remove some trophoblastic cells for genomic testing.
Clinical applications
Chorionic villus sampling (CVS) may be performed for the purpose of antenatal genetic or genomic diagnostic testing for the following indications:
- structural anomaly;
- high-risk screening for common aneuploidy;
- previous pregnancy affected by a chromosomal or genetic condition; and
- family history of certain chromosomal or genetic conditions.
Procedure
- CVS is performed between 11 and 13 weeks’ gestation. It can be performed transabdominally or transcervically, though in practice the latter is rarely required.
- A detailed ultrasound scan is performed to assess fetal lie, position and viability.
- The abdomen is cleaned with an antiseptic solution and the position of the placenta is identified.
- Local anaesthetic is usually offered and infiltrated in the likely path of the biopsy needle.
- A fine needle is passed through the mother’s abdomen into the uterus and a small biopsy of the placenta is removed under ultrasound guidance.
- Fetal wellbeing is confirmed following the procedure.
See Figure 1 below for an illustration of the transabdominal procedure.
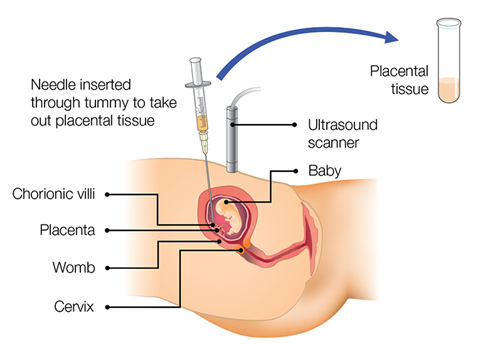
Figure 1: Chorionic villus sampling transabdominal method
Image taken from Public Health England: Screening in pregnancy: CVS and amniocentesis information for parents. Information from this website is licensed under the Open Government License v3.0.
Risks of CVS
- Miscarriage: the chance of having a miscarriage following CVS is estimated at around 1 to 2 per 200 tests performed (or 0.5%–1%) for a singleton pregnancy. The chance may be higher in twin or multiple-order pregnancies, at around 1 per 50 tests performed (or 2%).
- Where CVS is performed later in pregnancy, it may result in iatrogenic preterm labour or membrane rupture.
- Infection: the rate of severe infection for CVS is lower than 1-in-1,000.
Other considerations
Patients tend not to describe CVS as painful, but they do usually describe it as uncomfortable.
CVS is usually straightforward, but it can be a technically difficult procedure if there is a posterior or tricky-to-access placenta. In these circumstances, maternal bladder voiding may improve access, or it may be preferable to wait until amniocentesis is possible. If there is insufficient access to the placenta initially, it may be possible to successfully preform a CVS procedure in a week or two, so appointments can be rescheduled.
It is critical that pre-procedure counselling includes discussion about the risks outlined above in order to enable patients to make an informed decision about whether to proceed.
Alternative testing may include amniocentesis or non-invasive prenatal testing (this may include non-invasive prenatal screening or non-invasive prenatal diagnosis).
Rhesus status
Maternal rhesus status must be confirmed prior to CVS so that anti-D immunoglobulin can be given offered to those patients who are rhesus D negative.
Resources
For clinicians
- Royal College of Obstetricians and Gynaecologists: Amniocentesis and chorionic villus sampling (Green-top Guideline no. 8)
References:
- Ghi T, Sotiriadis A, Calda P and others. ‘ISUOG practice guidelines: Invasive procedures for prenatal diagnosis‘. Ultrasound in Obstetrics and Gynaecology 2016: volume 48, pages 256–268. DOI: 10.1002/uog.15945