Manchester (Evans) score
A scoring system that allows for calculation of the probability of pathogenic variants in the BRCA1 and BRCA2 genes.
Introduction
A number of models have been developed to try to predict the likelihood of identifying an underlying pathogenic variant in BRCA1/BRCA2 in an individual with a personal and/or family history of cancer. Such models include BOADICEA (now superseded by CanRisk), BRCAPRO, and the Manchester Score, developed by Evans and colleagues.
The Manchester Score was originally developed as a ‘back of the envelope’ calculation, with points awarded for each family member diagnosed with cancer (Table 1). The total score correlates with the probability of identifying an underlying pathogenic variant in either BRCA1 or BRCA2. A 10% ‘a priori’ probability of identifying such a variant is generally accepted to be the threshold at which constitutional (germline) genetic testing should be offered in the UK. Using the Manchester Score, this approximates as:
• a combined pathology adjusted score of 15 or more; or
• a single gene pathology adjusted score of 10 or more.
Score overview
Table 1: Familial cancer types and age at diagnosis
Points are awarded for each individual (including the proband) affected by cancer on the same side of the family (i.e. paternal or maternal), with different points awarded based on cancer type and age at diagnosis.
| Familial cancers | BRCA1 score | BRCA2 score | Combined score |
| Female breast cancer less than 30 years | 6 | 5 | 11 |
| Female breast cancer 30–39 years | 4 | 4 | 8 |
| Female breast cancer 40–49 years | 3 | 3 | 6 |
| Female breast cancer 50–59 years | 2 | 2 | 4 |
| Female breast cancer 60 years or more | 1 | 1 | 2 |
| Ovarian cancer 59 years or less | 8 | 5 | 13 |
| Ovarian cancer over 60 years | 5 | 5 | 10 |
| Male breast cancer 59 years or less | 5 | 8 | 13 |
| Male breast cancer over 60 years | 5 | 5 | 10 |
| Prostate cancer 59 years or less | 0 | 2 | 2 |
| Prostate cancer over 60 years | 0 | 1 | 1 |
| Pancreatic cancer | 0 | 1 | 1 |
Table 2: Proband tumour characteristics
As knowledge regarding the phenotype in carriers of pathogenic BRCA1/BRCA2 variants has improved, the score has evolved over time to avoid inappropriately awarding points to mucinous/borderline ovarian tumours, and to increase points awarded for breast cancers demonstrating a triple-negative (ER-negative/PR-negative/HER2-negative) phenotype.
The current iteration of the score allows adjustment to the score based on phenotypic features of cancer occurring in the proband, and attempts have been made to account for lack of available biological family history in adopted individuals.
| Characteristics of proband tumour (breast) | BRCA1 score | BRCA2 score | Combined score |
| HER2-positive | -6 | 0 | -6 |
| Lobular type | -2 | 0 | -2 |
| DCIS | -1 | 0 | -1 |
| Grade 1 | -2 | 0 | -2 |
| Grade 3 | 2 | 0 | 2 |
| ER-positive | -1 | 0 | -1 |
| ER-negative | 1 | 0 | 1 |
| Triple-negative | 4 | 0 | 4 |
| Characteristics of proband tumour (ovarian) | BRCA1 score | BRCA2 score | Combined score |
| Mucinous, germ cell, or borderline tumours | 0 | 0 | 0 |
| High grade serous under 60 years | 2 | 0 | 2 |
| Adopted individual (no known status in blood relatives) | 2 | 2 | 4 |
Worked example
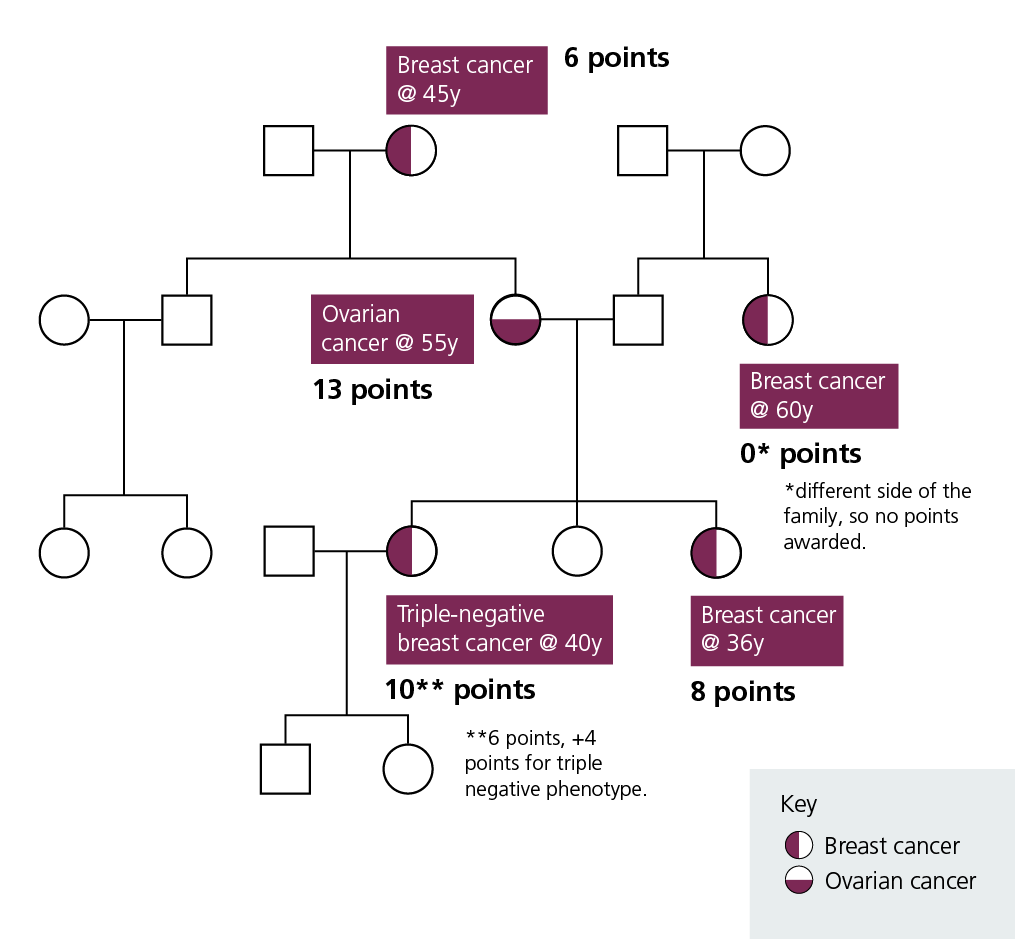
Figure 1: Worked example with a total combined Manchester score of 37 (10+8+13+6)
Offering testing
As per the current NHS National Genomic Test Directory, testing can be offered to:
- individuals affected by breast and/or high grade ovarian cancer with a combined pathology-adjusted Manchester score of 15 or more or single gene pathology adjusted score of 10 or more; or
- individuals affected by pancreatic cancer or prostate cancer with a combined pathology-adjusted Manchester score of 15 or more; or
- in families where there is no living affected relative to whom testing can be offered, unaffected first-degree relatives of an affected deceased individual with an estimated pathology-adjusted Manchester score of 20 or more.
Key messages
- The Manchester (Evans) score allows for calculation of the probability of pathogenic variants in the BCRA1 and BRCA2 genes using information about family members’ cancers.
- The type of cancer and age at diagnosis along with tumour characteristics are given points to make the calculation.
- Genomic testing is offered to patients with BRCA-associated cancer with a total score of 15 or more.
- Genomic testing is offered to unaffected first-degree relatives of a deceased individual with a score of 20 or more.
Resources
For clinicians
References:
- Evans DG, Eccles DM, Rahman N and others. ‘A new scoring system for the chances of identifying a BRCA1/2 mutation outperforms existing models including BRCAPRO‘. J Med Genet 2004: volume 41, issue 6, pages 474–480. DOI: 10.1136/jmg.2003.017996
- Evans DG, Harkness EF, Plaskocinska I and others. ‘Pathology update to the Manchester Scoring System based on testing in over 4000 families‘. J Med Genet 2017: volume 54, issue 10, pages 674–681. DOI: 10.1136/jmedgenet-2017-104584
- Evans DG, Lalloo F, Wallace A and others. ‘Update on the Manchester Scoring System for BRCA1 and BRCA2 testing‘. J Med Genet 2005: volume 42, issue 7. DOI: 10.1136/jmg.2005.031989