Progressive cardiac conduction disease
Progressive cardiac conduction disease is also known as young-onset idiopathic conduction disease. The condition presents with progressive anomalies of the electrical impulse generation and/or propagation affecting the sinus node, atrioventricular node and His-Purkinje system. It can result in life-threatening cardiac arrhythmia.
Clinical features
Patients may present with symptoms caused by bradycardia (a low heart rate), including dizziness or syncope. Life-threatening bradycardia may result in cardiac arrest.
With inherited disease, patients typically present with early-onset (<40 years of age) conduction anomalies. Electrocardiogram (ECG) findings may include prolonged P-wave duration, different degrees of atrioventricular block and bundle branch block patterns.
This article refers to cardiac conduction disease in the absence of structural heart disease and skeletal myopathy, so cardiac imaging, including echocardiography and cardiac MRI, will classically be within normal limits.
Genetics
SCN5A is the main gene associated with familial isolated progressive cardiac conduction disease (PCCD). It encodes the alpha unit of a sodium channel expressed primarily in the heart.
Variants in the gene NKX2.5 are often seen in association with congenital heart disease as well as in PCCD, but have also been found in isolated cases. The same is true of genes such as LMNA, GLA and DES in that the disease may start with isolated PCCD, although it tends to progress with an associated cardiomyopathy phenotype. The genes LAMP2 and PRKAG2 are metabolic causes of cardiomyopathy but may also present with isolated conduction disease.
Inheritance and genomic counselling
Cardiac conduction disease is genetically heterogeneous and phenotypic expression may vary within families. It usually has an autosomal dominant mode of inheritance, which means that there is a 50% chance of an affected individual passing it on to each child. Taking a three-generation family history and identifying any previously known familial pathogenic variants is important for genetic counselling. The testing criteria for PCCD can be found in the National Genomic Test Directory.
Management
Management of PCCD depends on the clinical presentation, but may include the following.
- Systematic exclusion of causes of isolated atrioventricular block is recommended before considering a genetic diagnosis (for example, sarcoidosis or Lyme disease).
- Indications for implantation of a permanent pacemaker typically follow the established guidelines for acquired conduction disease. This includes implantation for second-degree Mobitz type II and complete AV block, or symptomatic bradycardia (for example, syncope with clear heart rhythm-symptom correlation).
- Surveillance may be performed if the individual is asymptomatic and does not meet established guidelines for pacemaker implantation.
- An implantable cardioverter-defibrillator may be considered in certain situations (such as conduction disease associated with a LMNA or NKX2.5 pathogenic variant).
- First-degree relatives of an affected individual should be offered clinical screening. If genomic testing identifies a clear pathogenic variant, cascade family screening can be performed.
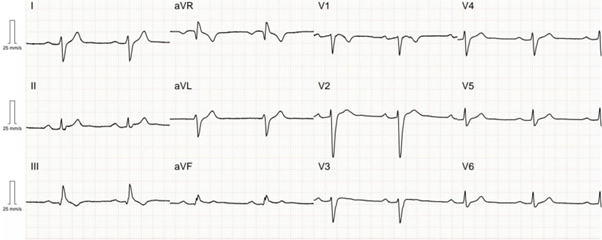
Figure 1: ECG from a patient with an SCN5A pathogenic variant demonstrating first-degree AV block and intraventricular conduction delay
Figure 1 shows an ECG from a patient with an SCN5A pathogenic variant demonstrating first-degree AV block (PR interval 260 ms) and intraventricular conduction delay (QRS duration 138 ms). The patient also developed sinus node disease with a 4.4 sec sinus pause and non-sustained ventricular tachycardia captured on an implantable loop recorder device.
Resources
For clinicians
- NHS England: National Genomic Test Directory
References:
- Glikson M, Nielsen JC, Kronborg MB and others. ‘2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA)‘. European Heart Journal 2021: volume 42, issue 35, pages 3,427–3,520. DOI: 10.1093/eurheartj/ehab364
- Wilde AAM, Semsarian C, Márquez MF and others. ‘European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases‘. EP Europace 2022: volume 24, issue 8, pages 1,307–1,367. DOI: 10.1093/europace/euac030
For patients
- British Heart Foundation: Progressive cardiac conduction defect
- Cardiac Risk in the Young: Progressive cardiac conduction defect