National Genomic Test Directory
The National Genomic Test Directory sets out the genomic tests that are commissioned by NHS England for patients with rare and inherited diseases and cancer. It covers the full repertoire of genomic testing technologies, from targeted testing through to whole exome and whole genome sequencing.
Overview
The test directory was established to bring the benefit of advances in genomics to NHS patients. Published in 2018, it built on the legacy of the UK Genetic Testing Network and the 100,000 Genomes Project.
The aims of the test directory are to:
- clearly outline the full range of genomic tests that are funded by the NHS in England;
- provide details about who is eligible for testing;
- specify the technology by which these tests are conducted;
- list the clinicians who are authorised to order each test;
- improve equity of access to genomic testing across the country; and
- ensure, through regular updates, that patients and families benefit from the very latest developments in our understanding of genomics.
How do I access the test directory?
The test directory can be accessed via the NHS England website. When you access the website, you will see this page (figure 1).
Figure 1: Screenshot of the National Genomic Test Directory webpage
(View larger size)
The three test directory documents
Genomic testing is offered both for the investigation of rare and inherited conditions, and for the investigation of cancer. There are three key documents to consider:
- National genomic test directory for rare and inherited disease. This spreadsheet outlines all the genomic tests commissioned by the NHS in England for rare and inherited disorders.
- Rare and inherited disease eligibility criteria. This document sets out the tests, and also which patients are eligible for each test. This document also specifies which health professionals from which specialties can request testing.
- National genomic test directory for cancer. This spreadsheet outlines the genomic tests commissioned by the NHS in England for cancer.
National genomic test directory for rare and inherited disease
The test directory for rare and inherited disease is grouped by different clinical indications. Each clinical indication (CI) is given a unique ID. In this document, CI IDs begin with ‘R’.
Some of the indications are narrow and specific, such as diagnostic testing for cystic fibrosis. Others are designed to be used where a specific diagnosis isn’t identifiable clinically and it makes sense to cast a wide net to maximise the chance of a diagnosis, such as testing for intellectual disability (large panel).
Rare and inherited disease eligibility criteria
The eligibility criteria document is often the best starting point when thinking about genomic testing to investigate rare and inherited disease as it is designed to support you in finding the appropriate test for your clinical need.
The contents of the document are divided into manageable sections based on clinical specialty. Under each section, there is a list of clinical indications; each with its CI ID (beginning with ‘R’) and a page number. When you find what you are looking for, click on the name or the CI ID and it will take you to the correct page.
National genomic test directory for cancer
The test directory for cancer outlines the genomic tests for cancer and is organised by tumour type (figure 2).
Within the document, there are separate sheets (accessed by tabs at the bottom of the page) for different tumour categories, for example, solid, neurological, paediatric, and so on.
Testing is grouped under the relevant CI, such as colorectal carcinoma. There is also information outlining the CI ID (in this document the ‘CI code’, beginning with ‘M’), the test code and test name, the target gene, the test scope, the technology used and the eligibility criteria.
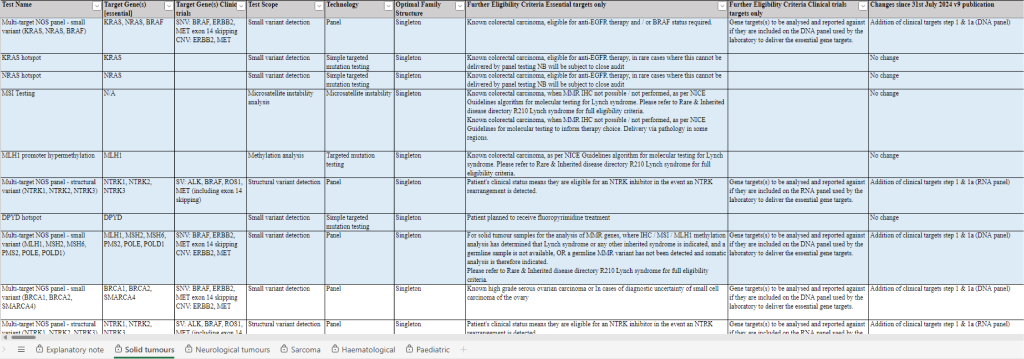
Figure 2: Screenshot of the cancer test directory
(View larger size)
A note on somatic (tumour) and constitutional (germline) testing for cancer
The test directory for cancer details somatic tests – those that look at pathogenic variants within the tumour DNA – whereas the test directory and eligibility criteria for rare and inherited disease cover constitutional tests to investigate genomic predisposition to cancer in an individual. For example, if you wanted to test for constitutional (germline) BRCA1 variants, then you would refer to the test directory for rare and inherited disease, not the test directory for cancer.
Test ordering
Local processes should be followed when requesting genomic tests. All referrals for testing will be triaged by the local Genomic Laboratory Hub (GLH) to ensure the most appropriate test is performed.
- Those requesting a genomic test will be asked to include the CI name and code, which can be found in the test directory as described above.
- When testing is requested by the clinical indication, the GLH will review the test request and relevant clinical information to check that the appropriate CI ID/code (for example, M1) has been selected.
- It is always helpful to include the decimal point to specify the constituent test(s) requested (for example, M1.7). If this information is not provided, the laboratory will attempt to complete the most appropriate testing based on the clinical detail given.
- Genomic testing should be targeted at those for whom genetic or genomic analysis will guide management, for example in aiding treatment selection or considering wider family testing.
Updates to the test directory
The test directory is regularly updated, as overseen by the Genomics Clinical Reference Group, who are supported and advised by expert test evaluation working groups. These working groups include scientific and clinical experts, patients and public representatives. The working group recommends additions and updates to the test directory.
Key messages
- The National Genomic Test Directory sets out the genomic tests that are commissioned by NHS England for patients with rare and inherited diseases and cancer in England.
- There are three key documents to consider:
- The national genomic test directory for rare and inherited disease.
- The rare and inherited disease eligibility criteria.
- The national genomic test directory for cancer.
- Local processes should be followed when requesting genomic tests. The local GLH will triage requests to ensure that appropriate testing is carried out.
Resources
For clinicians
-
- NHS England: National Genomic Test Directory
- NHS England Genomics Education Programme (GEP): Genomics in the NHS: A Clinician’s Guide to Genomic Testing in Rare Disease