Introducing the National Genomic Research Library
The NGRL houses data from patients and family members to support thousands of research projects. It enables approved researchers to access pseudonymised genomic data, health data and samples. This means that any personal data is changed so it can no longer identify an individual: names and other identifying information are removed and replaced with an individual reference number.
Establishing a unique data resource, with appropriate consent, will maximise the potential for scientific discovery, and the benefits of this will feed back into the NHS. Appropriate partnerships will increase the possibility of finding a diagnosis for patients, as well as developing new treatments, diagnostics and tools for discovery.
The NGRL will initially only apply to patients with rare diseases and cancers who are eligible for WGS in the GMS. The offer to participate is considered part of the standard clinical pathway for patients, as information found via research can be fed back into clinical care. Participation is optional, however. Individuals can decide not to take part, defer their decision, or withdraw at any time.
Governance of Genomics England and the NGRL
The NGRL is a national initiative with support from NHSE/I, Genomics England, and the Department of Health and Social Care (DHSC). Genomics England is a company wholly owned by the DHSC, set up initially to deliver the 100,000 Genomes Project.
The protocol and supporting materials have been approved by a research ethics committee governed by the Health Research Authority, and do not require local ethics approvals. The data sharing and access arrangement in the NGRL adheres to the principles and data information rights of the General Data Protection Regulation, the Information Commissioner’s Office and the National Data Guardian.
Typically, research studies are offered to patients individually, each with their own unique protocol and consent process. The NGRL, however, is similar to a biobank and the research offer within the 100,000 Genomes Project, and so involves one consent process to enable patient samples and data to be stored and accessed by a number of researchers.
The NGRL is not part of the National Institute for Health Research Clinical Research Network Portfolio of studies. It is not a clinical trial and Good Clinical Practice training is not mandatory, although it may be encouraged as it covers key principles of clinical research.
Implications for the patient
Potential impact of the NGRL
For patients with certain rare diseases, contributing to the NGRL can increase the likelihood of finding a diagnosis, as researchers have a larger amount of variant data to compare and help determine whether genetic variants cause or are associated with different health conditions. Or, for patients with cancer, they may be found to be eligible for a clinical trial or treatment in the future.
Contributing to the NGRL may not directly help each individual patient but could help others in the future. Having a larger data set can allow for more information to be learned about rare diseases and cancers, by being able to compare genomic and health information about patients nationally and internationally. As researchers use the data to learn more about health conditions, findings can be used to help guide clinicians in the management of patients with these conditions, or to develop drugs and diagnostic tools where the development work is typically done outside of the NHS.
Members of the Genomics England Participant Panel, made up of rare disease and cancer patients and their families, support the view that while a therapy or cure may not be immediately available for a patient’s condition, the chances of finding one are greatly increased by participating in the NGRL. This can help to transform personalised medicine for patients, their families and for wider society.
Participants from the 100,000 Genomes Project have also shared their experiences through a series of films that demonstrate the impact that whole genome sequencing and research can have for patients and their families.
Future recontact of the patient
Certain approved staff within Genomics England will be able to see both the identified and de-identified patient data in order to enable opportunities for patients to receive information about their diagnosis or to access a clinical trial that was identified by research, through an approved policy.
Patients who choose to contribute to the NGRL may be contacted in the future for:
- Possible diagnosis: If a potential diagnosis of a rare disease is made by a researcher for an individual or group of individuals, they will notify Genomics England based on the unique identification number given to each patient. Genomics England can determine the identity of the patients and contact the relevant GLH and/or clinical teams involved to discuss the findings and confirm them in a clinically certified laboratory. Typically, the clinical team would then contact the patient if the findings are confirmed to be relevant.
- Additional samples: On occasion, further samples may be requested for specific research projects. In some cases, separate consent for certain samples may be required, and patients can decide whether they wish to do this. If the patient was determined to be eligible for a specific national or local research study or clinical trial, they would also need to go through a separate consent process as per the study’s protocol.
- Reviewing consent: Patients may be contacted to review their decision to take part in the NGRL. For example, this would be required once an individual reaches the age of 16, if they were initially included in the NGRL as a child with the decision made by their parent or guardian.
- Updates and opportunities: Occasionally, approved staff from Genomics England may contact patients directly, for example if there is general news about the NGRL and/or about research opportunities.
Patients will not be contacted for marketing purposes, as data access is not permitted for this reason. It is not feasible to inform individual patients when their data has been accessed, because it forms a part of millions of bits of information that are de-identified and may be reviewed in large data sets.
Use of data
Patient data in the NGRL
If a patient chooses to have WGS and contribute to the NGRL, they are giving permission to Genomics England to manage access to their data collected by the GMS. Data in the NGRL is pseudonymised, which means that identifiers such as names, dates of birth and contact details are removed, and each patient record is given a unique identification number.
For patients who choose to participate in the NGRL, this information is collected throughout their life and after their death, unless they choose to withdraw. Types of data in the NGRL include:
- data about the sample provided by the patient (such as blood, tissue and/or saliva), including information to track the sample location and testing process;
- data from the analysis of that sample, including the raw data from genomic tests, the variants found and the result of interpretation of these variants;
- clinical data about the patient, including the information about their condition that is submitted by the referring clinician via the GLH when WGS is requested; and
- secondary clinical data, including information from NHS, GP and social care records, and national disease registries that may not be related to the patient’s diagnosis. This also includes information from organisations including Public Health England or NHS Digital; for example, information about admissions, appointments and outcomes at NHS hospitals in England. Information from these sources is provided directly and therefore complies with their existing legal requirements and standards.
Genomics England may identify new sources of health data that would be important for research and added to the NGRL in the future. Any new data sources will undergo a robust review process prior to approval, ensuring compliance with NHS England standards and that it maintains the principle of participants remaining de-identified to researchers.
Use of data for clinical care versus the NGRL
If the patient declines to participate in the NGRL, it is also important to note that, as part of having a clinical WGS test, the patient’s clinical sample and genomic sequencing data can still be accessed by healthcare professionals and laboratory scientists across the NHS in England for the purpose of patient care.
Data can also be accessed by healthcare and laboratory professionals internationally, for example as part of laboratory accreditation schemes, when asking for an expert opinion as to the patient’s diagnosis, or sharing data to help improve the interpretation of the tests. This involves an additional process that removes patient identifiers.
Patients cannot opt out of this if they choose to proceed with WGS, and this is separate to the decision to allow their samples and data to be accessed as part of the NGRL.
Data access in the NGRL
Certain staff within Genomics England will be able to see both identifying and de-identified patient data, enabling them to ensure that the process by which researchers can access de-identified data is securely controlled, and that information found by researchers can be fed back to healthcare professionals if relevant for their patient.
Researchers that can access the NGRL could be from UK or other countries. They may work within the NHS, hospitals, universities or charities. They can also include private healthcare groups or commercial companies, such as pharmaceutical companies, that use data to understand how current medicines could be improved or how new drugs or tests could be developed to help patients.
All researchers are vetted by an oversight committee before being granted permission to access the data, which is held in a form that cannot be copied or removed without the permission of Genomics England, and may only be used for purposes that are in line with their acceptable use policy.
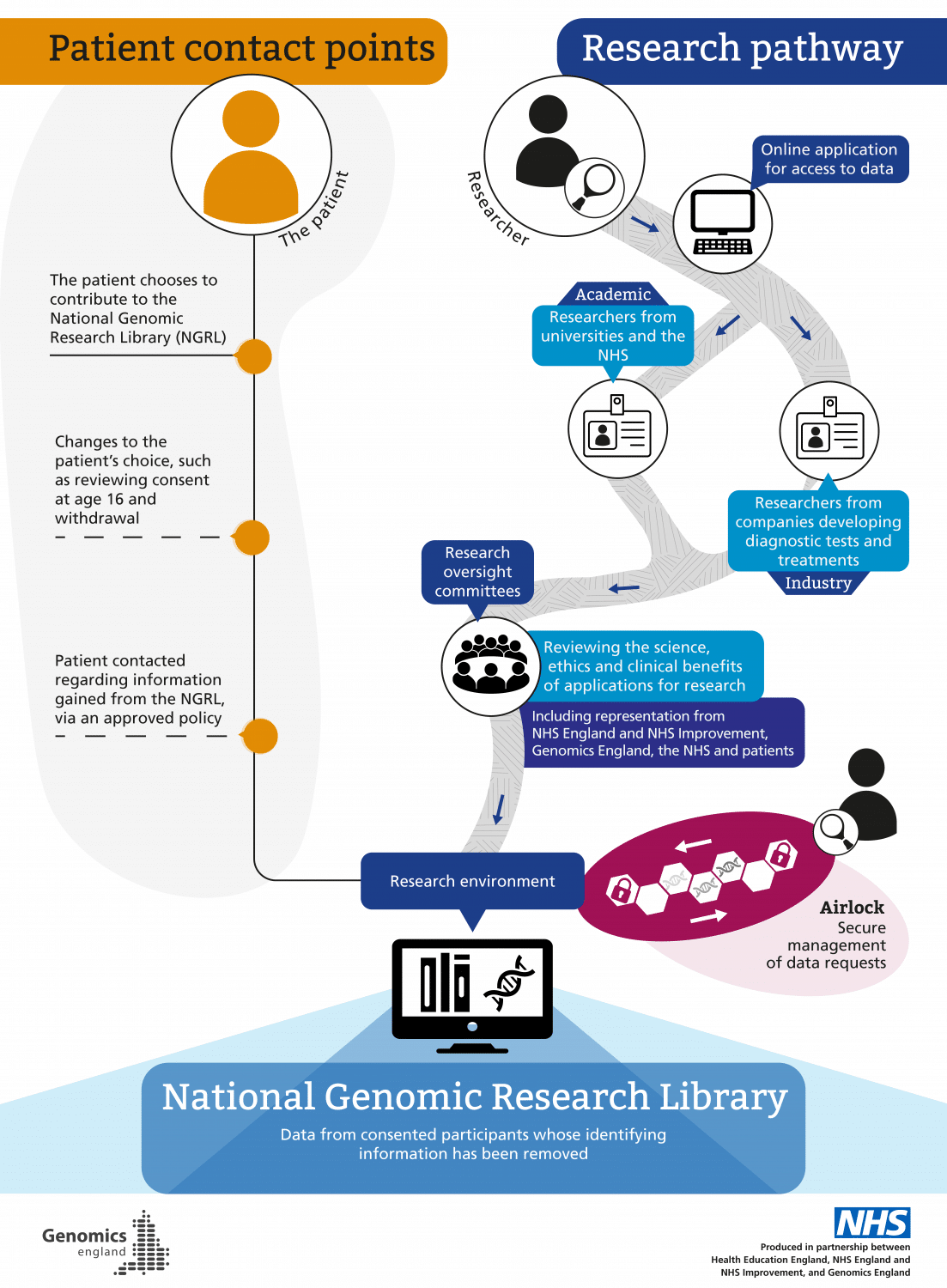
There is a robust governance process in place within Genomics England for accessing data in the NGRL (see graphic, ‘How data access is managed’ – click to enlarge).
Researchers may be from universities and the NHS, or from industry (companies developing diagnostic tests and treatments). Any researcher must be approved in order to collaborate with Genomics England and other partners, including feeding back about how data collection and analysis may be improved to support research.
Researchers’ identities are checked and confirmed, and they must submit a research proposal detailing how and why they want to use the data for health purposes. This is reviewed by research oversight committees, which review the science, ethics and clinical benefits of applications for research. These committees include representation from NHSE/I, Genomics England, the NHS and patients. Once approval is granted, read-only access to the data is provided via access to a secure server, and activity within this environment is monitored. This acts like a reference library, meaning researchers cannot copy or remove information in the NGRL.
Instead, researchers work with the data using various tools available within the research environment. Once they have analysed the data they need, this can be taken out of the environment following an approved ‘air lock’ process, which manages data requests to approve the transfer of analysed data out of the NGRL (such as for publication), as well as findings that can be added back into the NGRL to contribute to ongoing research. In general, only summary-level (rather than individual-level) de-identified data can be taken out of the research environment.
Data cannot be accessed by groups such as non-health related government agencies, including those linked to employment, insurance companies, police and border agencies, or for marketing purposes.
De-identifying data makes it very difficult for any researcher to identify individual patients, and they are made aware that doing so is illegal. Identification is not impossible because of the unique nature of each individual’s genomic code; for example, if a patient is already part of a patient group for a rare disorder and has granted access to genomic data via other research, those researchers that also have approved access to the NGRL could identify that individual.
How data access is managed
Data storage
Data is physically stored in secure UK data centres, which include facilities that also house other high-security government data sets including NHS data.
The data security within these data centres remains the responsibility of Genomics England.
Use of samples
When patients choose to have clinical WGS testing, their physical DNA samples are stored in their local GLH, and can be accessed by other laboratories within the GMS. Tumour samples may also be stored in their local hospital histopathology unit.
If the patient also chooses to contribute to the NGRL, they give permission for Genomics England to access their DNA, as well as additional tissue or other samples that the patient has stored in the NHS, if it is not otherwise needed and would not affect their care.
If further samples are required for research that cannot be obtained from existing samples, patients may be contacted by Genomics England to discuss separate consent to gain new samples for research purposes.
Samples are held securely within the UK. They would not be sent outside the UK without separate explicit consent.
Use of samples for clinical care versus the NGRL
If a patient declines to participate in the NGRL, their sample(s) will only be used for their clinical WGS test.
Broadly, the use of samples within a clinical setting versus a research one is an important distinction to note. For example, under Human Tissue Authority (HTA) guidance, clinical samples of fresh tissue (such as for cancers) can be taken for future clinical genomic testing as part of standard diagnostic care and prior to consent being specifically made. Tissue samples could not be utilised for any research purposes, however, including within the NGRL, prior to explicit consent.
Discussing the NGRL with patients
The offer to take part in the NGRL should be made to all patients being offered whole genome sequencing (WGS). It is anticipated that the timing of this discussion will be situation-dependent and may take place at different points in the patient’s clinical pathway, including at the time of requesting the clinical test, by separate telephone and/or email communication, at the time when results are given for the clinical test, or at a subsequent follow-up appointment.
Genomics England has developed online information for patients about the NGRL, which can be found here. As well, local pathways for discussing the NGRL along with offering clinical WGS will have been developed in your region; please contact your GLH for further information.
The conversation about the NGRL is not prescriptive and therefore the duration can range depending on the needs of the patient. As is standard practice with any research offer, patients should be supported to take as much time as they need to consider whether they wish to contribute to the NGRL. Information will be made available online for clinicians and patients to support these discussions.
Where appropriate (if the clinician knows that WGS will be offered to the patient), information can be sent or signposted to patients in advance of the consultation where the NGRL will be discussed. The GLH should be updated by the clinical team if the patient makes a decision about the NGRL at a later date than when the clinical test was requested.
Patients may decide that they do not want to contribute to the NGRL, or wish to have more time consider their choice. In these instances, it should be made clear that the clinical WGS test will continue to be processed if they consented to this.
The choice to contribute to the NGRL does not apply to any other NIHR portfolio, national or local research study. As well, if the patient does opt in, this choice applies to any future genomic tests they have within the Genomic Medicine Service, unless they decide to withdraw from the NGRL at any time.
If multiple members of a family are having WGS, each individual may make a different choice about whether or not to take part in the NGRL. If not all family members choose to take part, some data (for example, health records) will not be included in the research environment.
Members of the Genomics England Participant Panel have shared the considerations they made in making their decision about taking part in genomic research. You can listen to their interviews below, or view our Top Tips for discussing the NGRL with patients.
Recording patient choices about the NGRL
Each patient’s (and family member’s, where relevant) choices are captured via nationally standardised record of discussion (RoD) forms. For more information about recording patient choices, please see our page on requesting whole genome sequencing.
Withdrawing from the NGRL
Patients who choose to contribute to the NGRL should be aware that they can withdraw at any time. They would be required to confirm their choice via the withdrawal form, which outlines two different types of withdrawal:
- Partial withdrawal means that the patient is still happy for their data to continue to be stored and accessed in the NGRL for research, but they do not wish to have any further contact from Genomics England or their clinical team with regard to any information found in the NGRL that may be relevant to them.
- Full withdrawal means that the patient no longer wants their data to be stored and accessed in the NGRL, and does not want further contact. Health and genomic data is updated by Genomics England in the NGRL in regular ‘data releases’. For patients who request full withdrawal, their data will not be included in any future data releases for further research access, and limited information would only continue to be used by Genomics England for auditing purposes (that is, having a record of individuals who were once part of the NGRL and then chose to withdraw).
It is important to note that the patient’s data cannot be removed from research that has already taken place or is currently under way.
Further information
Resources to support patients
- Genomics England: information for patients considering participating in the NGRL
- Genetic Alliance: national charity for patients and families affected by genetic conditions
- SWAN UK: supporting families affected by a syndrome without a name
- Unique: understanding rare chromosome and gene disorders
- Macmillan Cancer Support
- Medline Plus Genetics: patient and public-friendly information about genetics and health conditions from the US National Library of Medicine
Information for clinicians
- NHSE National Genomic Test Directories
- GeneReviews: an international clinician resource providing information about inherited conditions
- Joint Committee on Genomics in Medicine’s Consent and Confidentiality in Genomic Medicine 2019 report